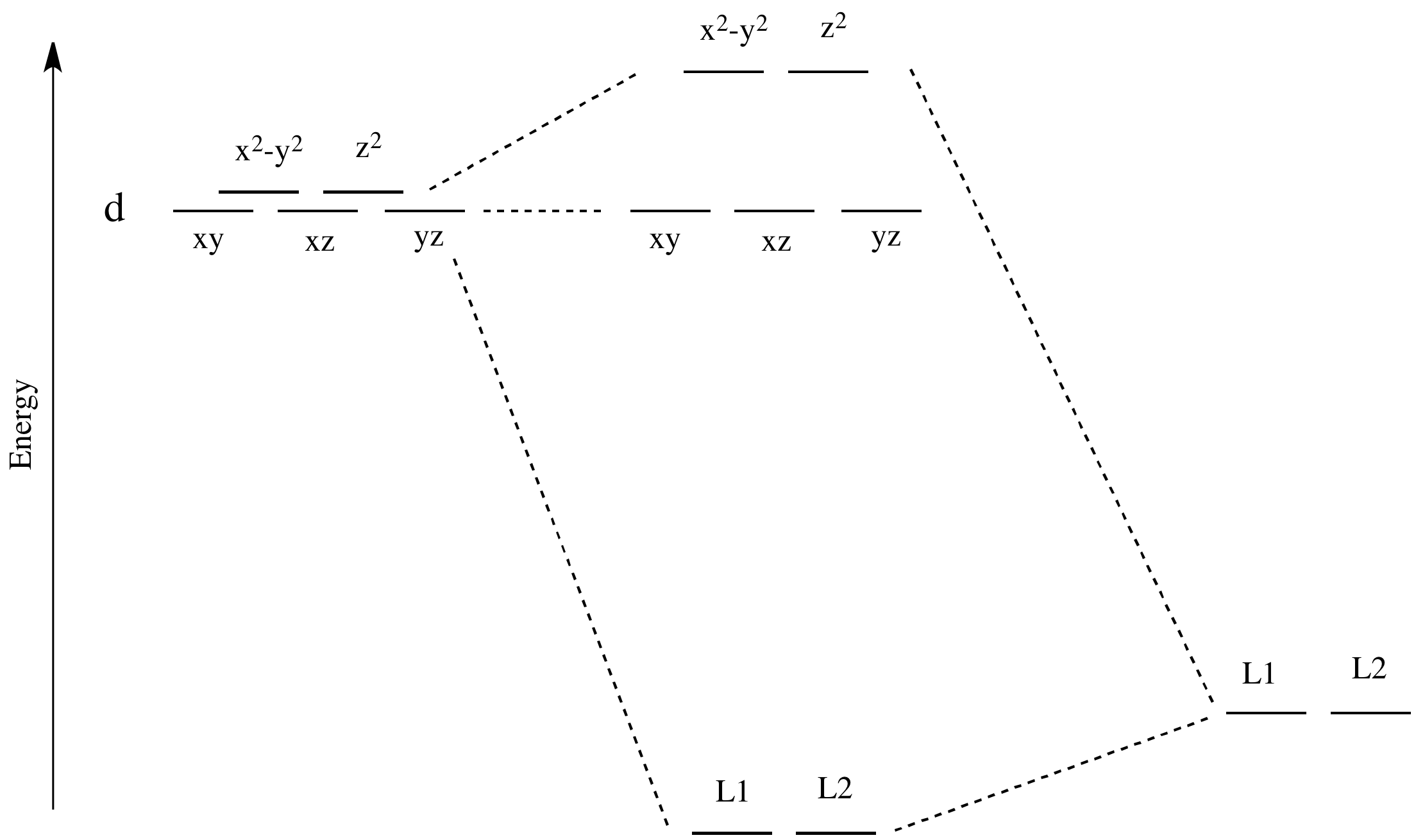
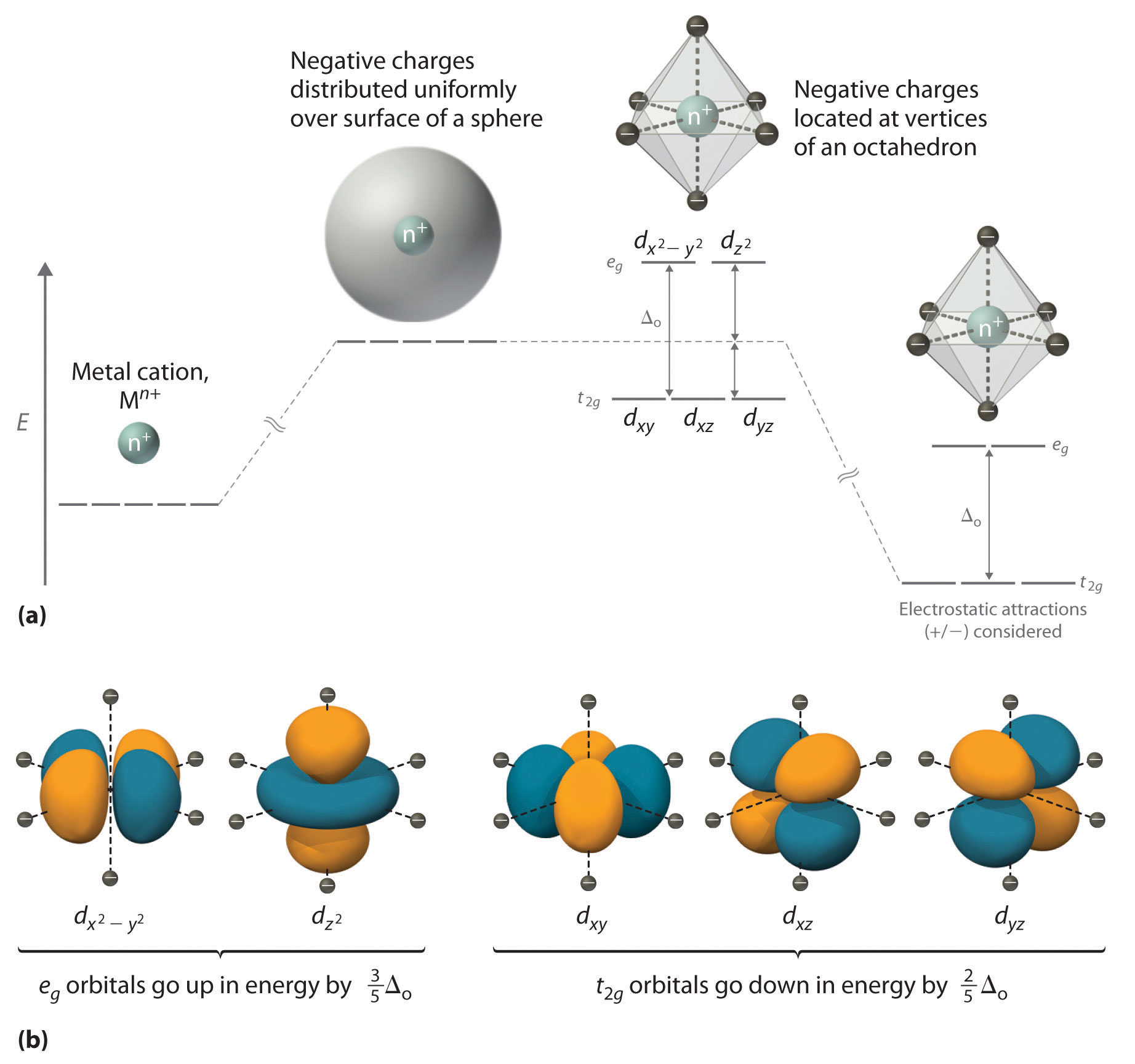
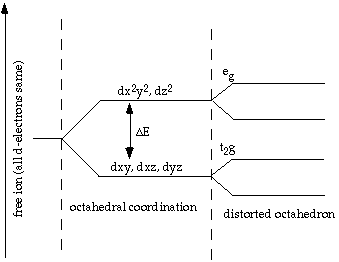
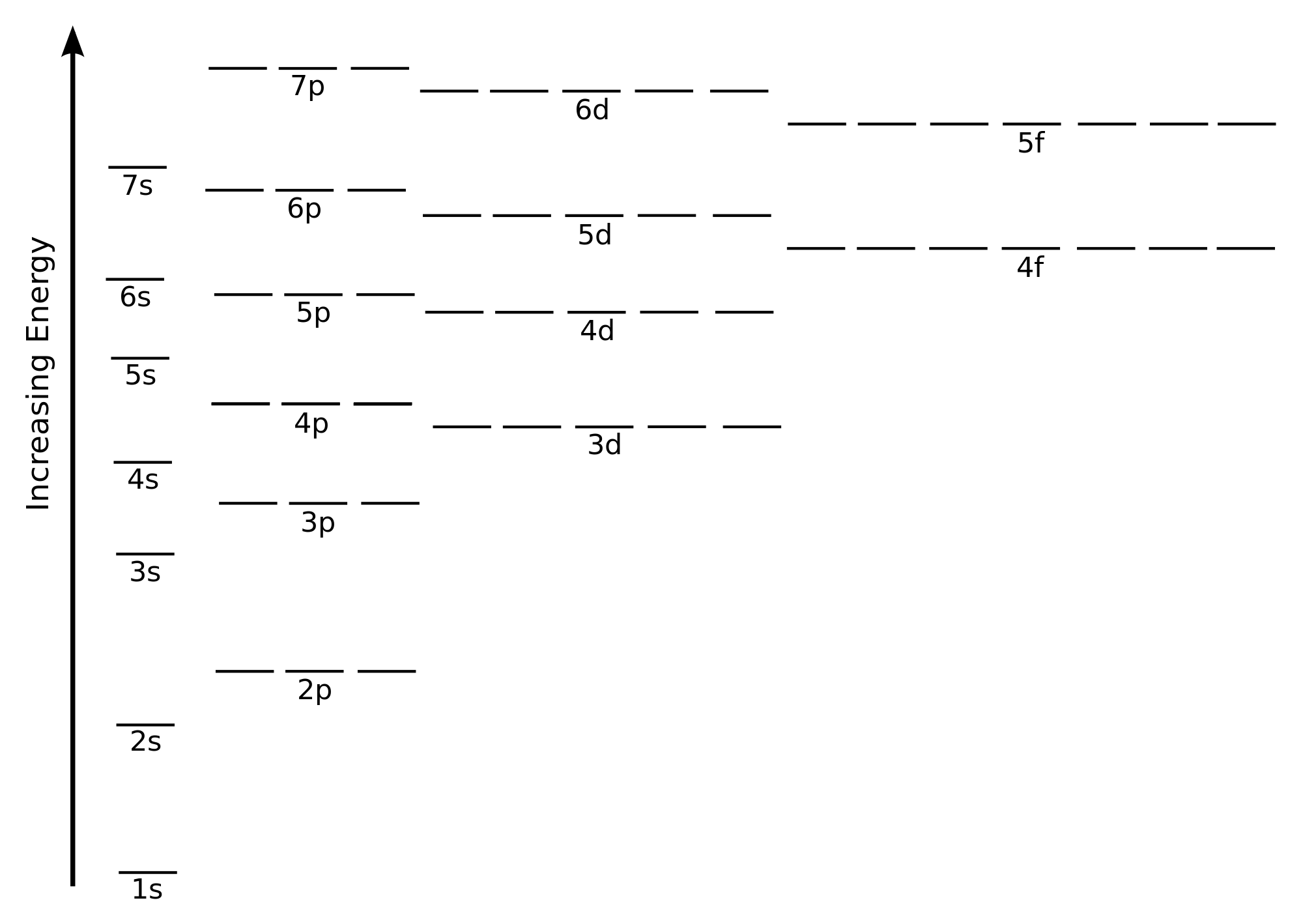
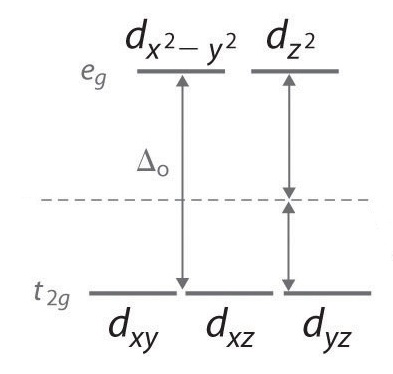
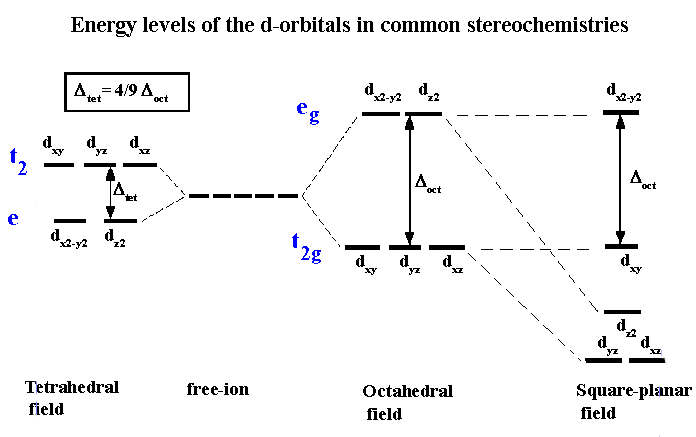
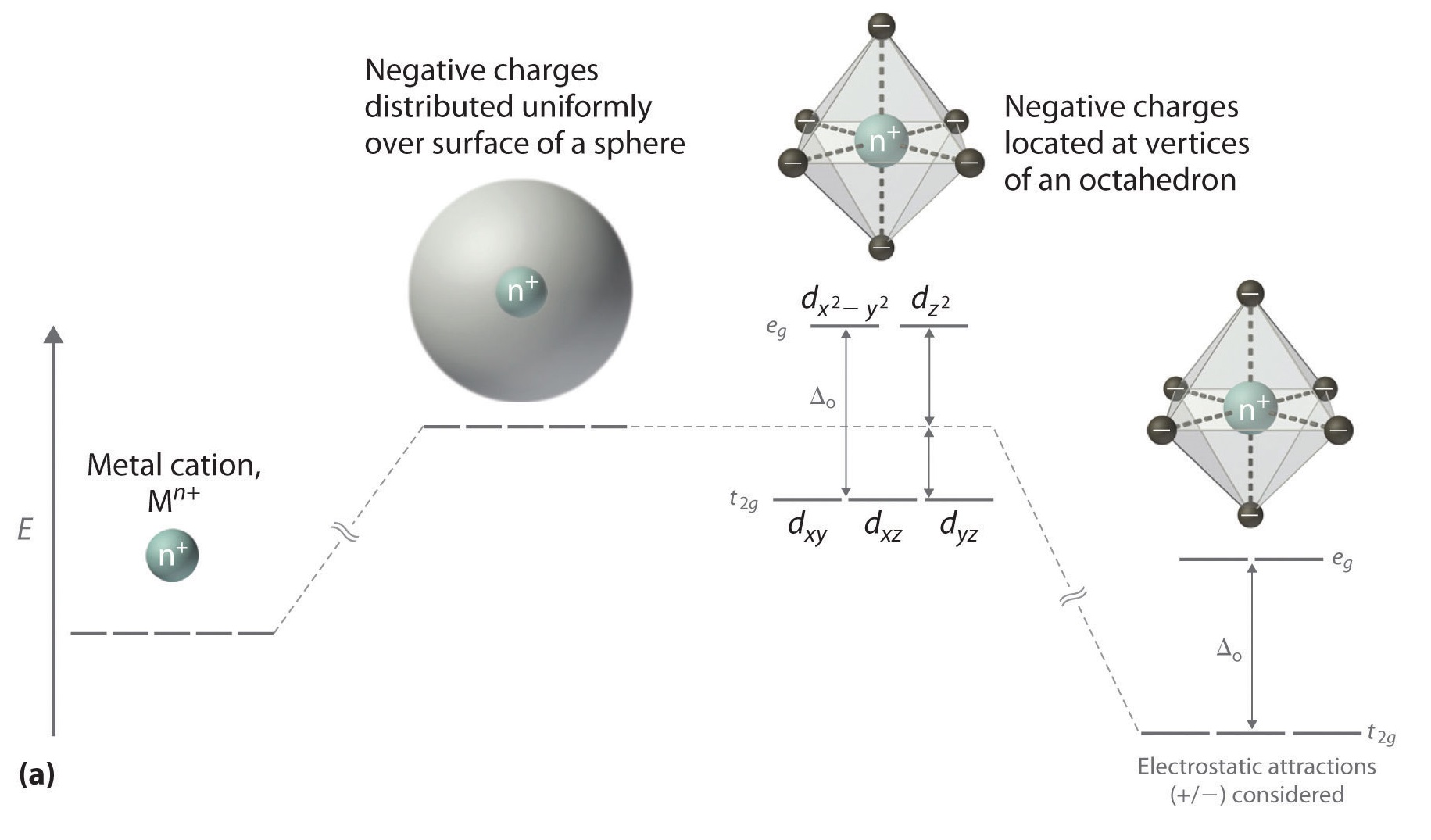
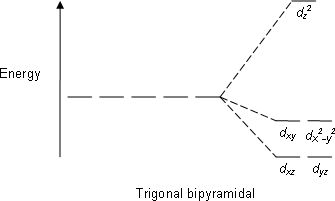
D Orbitals Energy Levels

In the n 1 shell you only find s orbitals in the n 2 shell you have s and p orbitals in the n 3 shell you have s p and d orbitals and in the n 4 up shells you find all four types of orbitals.
D orbitals energy levels. Orbitals orbital energy orbital energy level the energy of an electron in a single atom can be determined solely by the principal quantum number. There are 5 d orbitals in the d subshell. Orbitals are named as s p d and f. A p orbital can hold 6 electrons.
At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3p x 3p y 3p z. D and f orbitals. Calculating the energy level of an orbital. To see the elongated shape of ψ x y z 2 functions that show probability density more directly see pictures of d orbitals below.
It is important to note here that these orbitals shells etc. Based off of the given information n 4 and ℓ 3. The shapes of the first five atomic orbitals are. These spaces called orbitals are of different shapes denoted by a letter s p d f g.
It should be noted that the subshells are energy levels as well called subsidiary orbital energy levels so if we sort the subshells in ascending order in terms of their energy levels it would be s p d f. All levels except the first have p orbitals. Are all part of an empirical theory designed to explain what we observe with respect to molecular structure and bonding. The energy of that one.
The energy of atomic orbitals increases as the principal quantum number n increases. The 2s orbital would be filled before the 2p orbital because orbitals that are lower in energy are filled first. In a single electron hydrogen like atom the orbital energy i e. 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f.
Each picture is domain coloring of a ψ x y z function which depend on the coordinates of one electron. Energy levels are named as. At the third level there are a total of. Orbitals can be ranked in the increasing order of orbital energy as follows.
The 2s orbital is lower in energy than the 2p orbital. Energy levels are the electron shells that are located around the nucleus. In any atom with two or more electrons the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s p d f. In most cases only the electrons contained in the s and p orbitals are considered valence electrons electrons seek the lowest energy level possible.
An orbital is the most probable region where an electron can be found around the nucleus. Difference between orbitals and energy levels definition. Within each energy level is a volume of space where specific electrons are likely to be located. Thus there are 3 angular nodes present.
1s 2s 2p x 2p y and 2p z the two colors show the phase or sign of the wave function in each region.