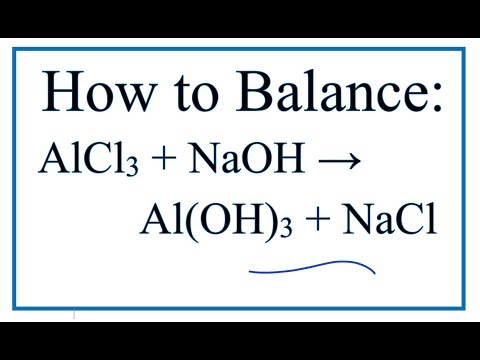
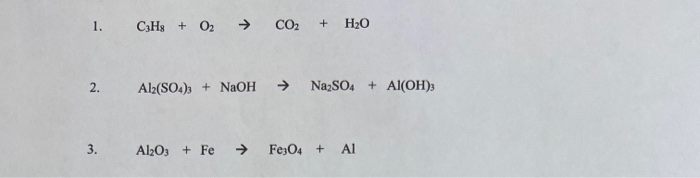Al2 So4 3 Naoh Al Oh 3 Na2so4 Balance
Balance the reaction of al2 so3 3 naoh na2so3 al oh 3 using this chemical equation balancer.
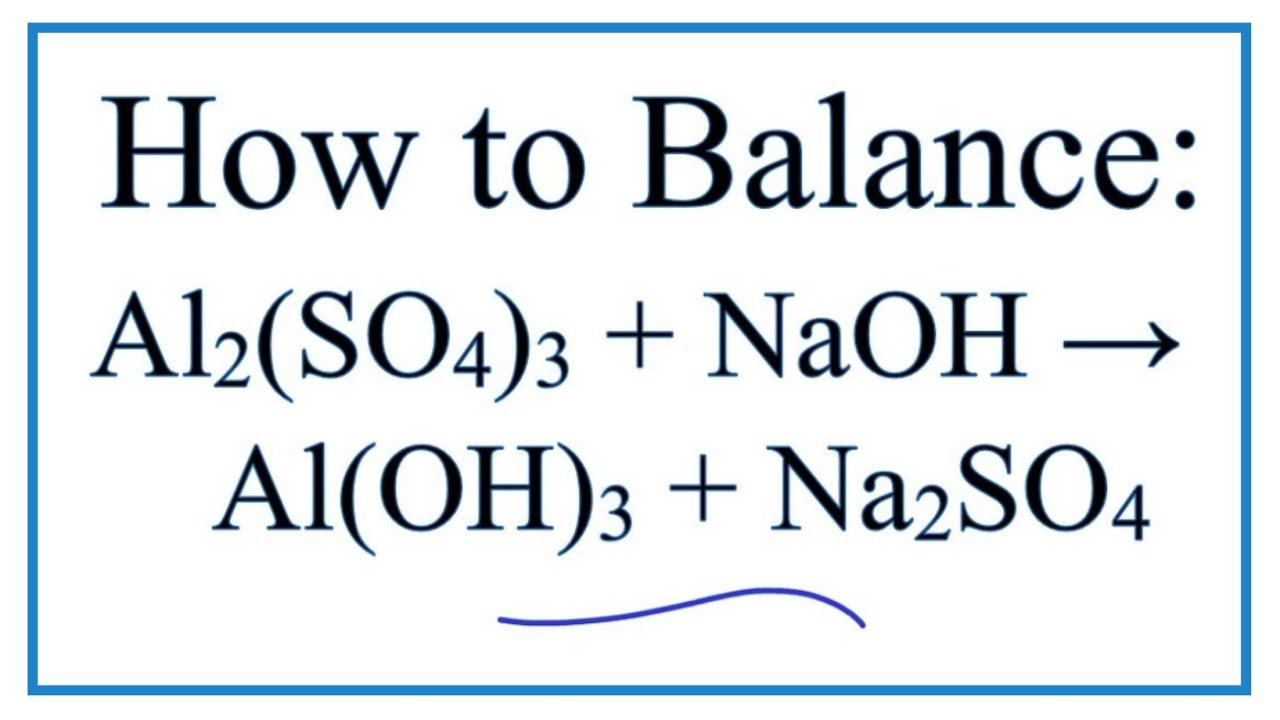
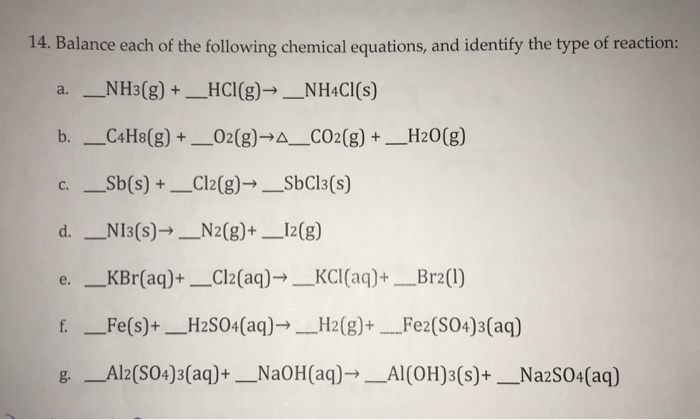
Al2 so4 3 naoh al oh 3 na2so4 balance. Of atoms on both side of the equation. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Balance the reaction of al2 so4 3 naoh al oh 3 na2so4 using this chemical equation balancer. If there are 2 al 3 so4 you equalize them to the right hand products so i would 1st get.
Sum of the coefficients is 12. Choose aluminum sulfate al2 so4 3 as the most complex. Al2 so4 3 6naoh 2al oh 3 3na2so4. Fe cl 2 fecl 3.
Al 2 so 4 3 6naoh 2al oh 3 3na 2 so 4 check the balance aluminium sulfate react with sodium hydroxide to produce aluminium hydroxide and sodium sulfate. Enter either the number of moles or weight for one of the compounds to compute the rest. Al2 so4 3 naoh al oh 3 na2so4 first of all equal the no. Now balance out naoh since on the right side of the equation there are 6 na and 6 oh and the final result is.
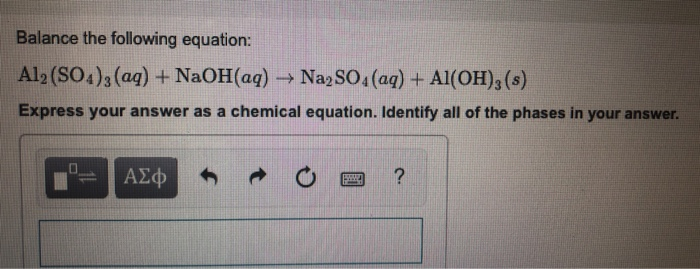
Examples of complete chemical equations to balance. Then you can add the coefficients. Reactants products al 2. Al2 so4 3 naoh al oh 3 na2so4 balance this equation 2 see answers wajahatkincsem wajahatkincsem answer.
Al2 so4 3 6 naoh 2 al oh 3 3 na2so4. To balance al2 so4 3 n. Reaction stoichiometry could be computed for a balanced equation. In this video we ll balance the equation al2 so4 3 naoh al oh 3 na2so4 and provide the correct coefficients for each compound.
The balanced chemical equation is. This is the balanced equation. Equation al2 so4 3 naoh na2so3 al oh 3 is an impossible reaction please correct your reaction or click on one of the suggestions below. Here is the chemical equation.
Al2 so4 3 naoh al oh 3 na2so4 instructions and examples below may help to solve this problem you can always ask for help in the forum.